Dühring's rule
Encyclopedia
Dühring's rule states that a linear relationship exists between the temperatures at which two solutions exert the same vapor pressure. The rule is often used to compare a pure liquid and a solution
at a given concentration
.
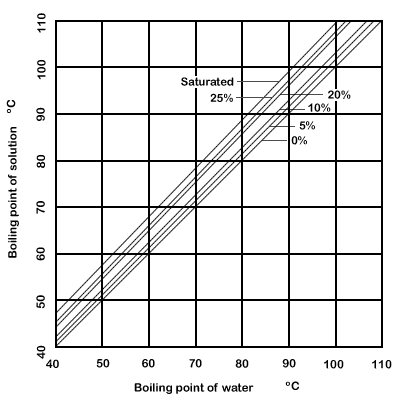
Dühring's plot is a graphical
representation of such a relationship, typically with the pure liquid's boiling point
along the x-axis and the mixture's boiling point along the y-axis; each line of the graph represents a constant concentration.
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
at a given concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
.
Dühring's plot is a graphical
Cartesian coordinate system
A Cartesian coordinate system specifies each point uniquely in a plane by a pair of numerical coordinates, which are the signed distances from the point to two fixed perpendicular directed lines, measured in the same unit of length...
representation of such a relationship, typically with the pure liquid's boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
along the x-axis and the mixture's boiling point along the y-axis; each line of the graph represents a constant concentration.


